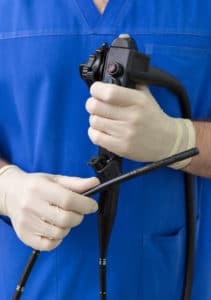
Each year, upward of 500,000 Americans undergo endoscopy procedures, which involve insertion of a flexible tube outfitted with a tiny light and camera used to capture images of a patient’s digestive tract. As patients, we trust that the medical devices used by healthcare providers are unquestionably safe, but for an increasing number of endoscopy patients worldwide, that trust has been irrevocably broken.
In 2012 inside a Dutch laboratory, a technician working for Tokyo-based medical device manufacturer Olympus Corp. peeled back the tip of a duodenoscope made by the company, revealing a brown, grimy film. Investigators confirmed that the film was the result of blood and tissue trapped inside several parts of the device that should have been sealed. Turns out the rubber ring designed to keep bacteria out had been cracked and worn. As a result, multiple patients at an area hospital had been sickened.
Over the next three years, 21 people died and dozens of other patients were infected in 10 outbreaks identified by the US Food and Drug Administration – seven of which involve Olympus scopes. Yet, throughout that time, the company, which controls some 85 percent of the US market for these devices, continued to sell them and failed to warn hospitals of the infection risk. Instead, officials blamed infection incidents on hospitals for failing to properly clean the devices.
Exacerbating the problem, FDA officials say, may be defective endoscope reprocessing devices made by Warminster, PA-based Custom Ultrasonics. The devices are designed to kill bacteria and microorganisms found on flexible endoscopes after hospital procedures, so that they can be re-used safely, but FDA inspectors repeatedly have deemed the devices ineffective in fully disinfecting endoscopes.
Both Olympus-made duodenoscopes and Custom Ultrasonics-made endoscope reprocessing devices currently are under recalls, with warnings to healthcare providers, patients and their families that the devices pose increase risk of serious bacterial infections including E-coli, klebsiella pneumoniae, pseudomonas aeruginosa and CRE (carbapenem-resistant enterobacteria), considered a superbug – strain of bacteria that has become resistant to antibiotic drugs.
If you or someone you love have suffered an infection, illness or death after undergoing an endoscopy procedure, call Jacksonville’s Harrell and Harrell at 800-251-1111 immediately to schedule a legal consultation with an attorney specializing in defective medical devices.
